EXAMPLES OF NMR SPECTRA OF OTHER ISOTOPES
If it happens that the molecule you are analyzing contains heteroatoms, i.e. atoms different from 1H and 13C, your recording of NMR spectra at the proper frequency of those heteronuclei can be very revealing concerning certain structural aspects.
The most useful heteroatoms are those bearing a magnetic moment I = ½ and a high natural abundance. This is the case of 19F and 31P both 100% abundant.
There are many pharmaceuticals and numerous molecules with biological interest containing 19F, 31P or both.
Look at the spectra of the molecule HOOC-C(CH3)3.
What would happen if one replaces a hydrogen of one of the three methyl groups by F?
The molecule would turn into HOOC-C(CH2F)(CH3)2 and the recording of a 19F-NMR spectrum would be then possible.
But, would 19F exert any effect over the 1H- and 13C-NMR spectra? Maybe you are going to be surprised...
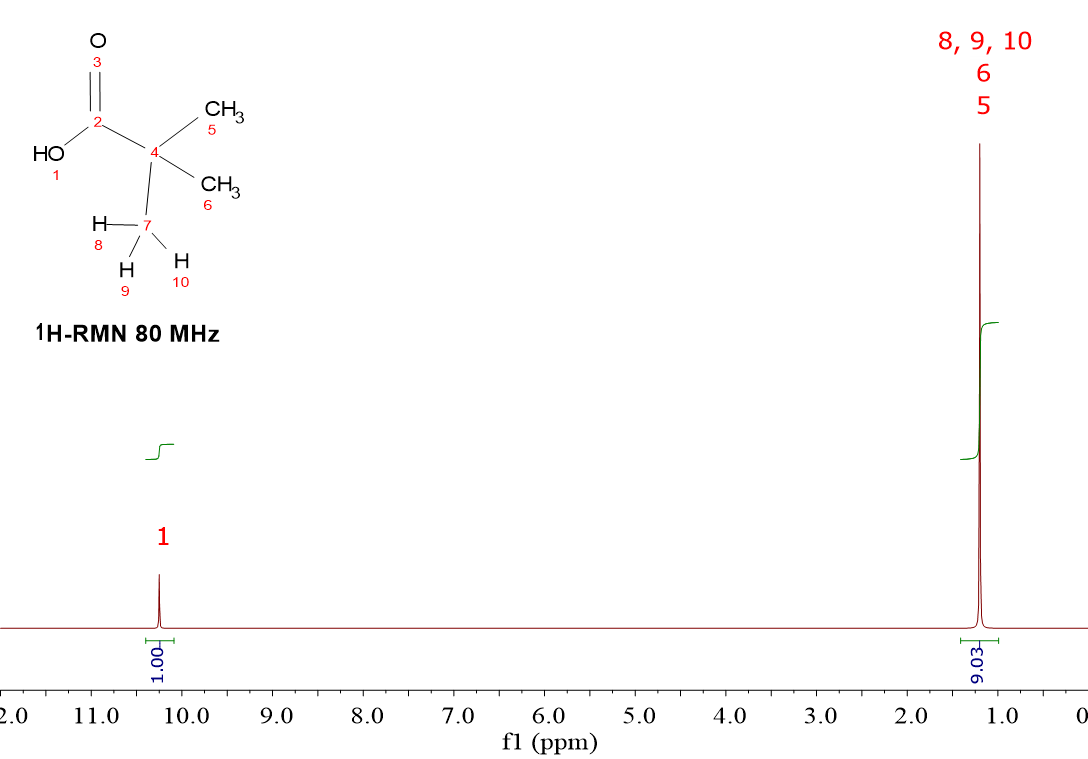 HOOC-C(CH3)3
HOOC-C(CH3)3
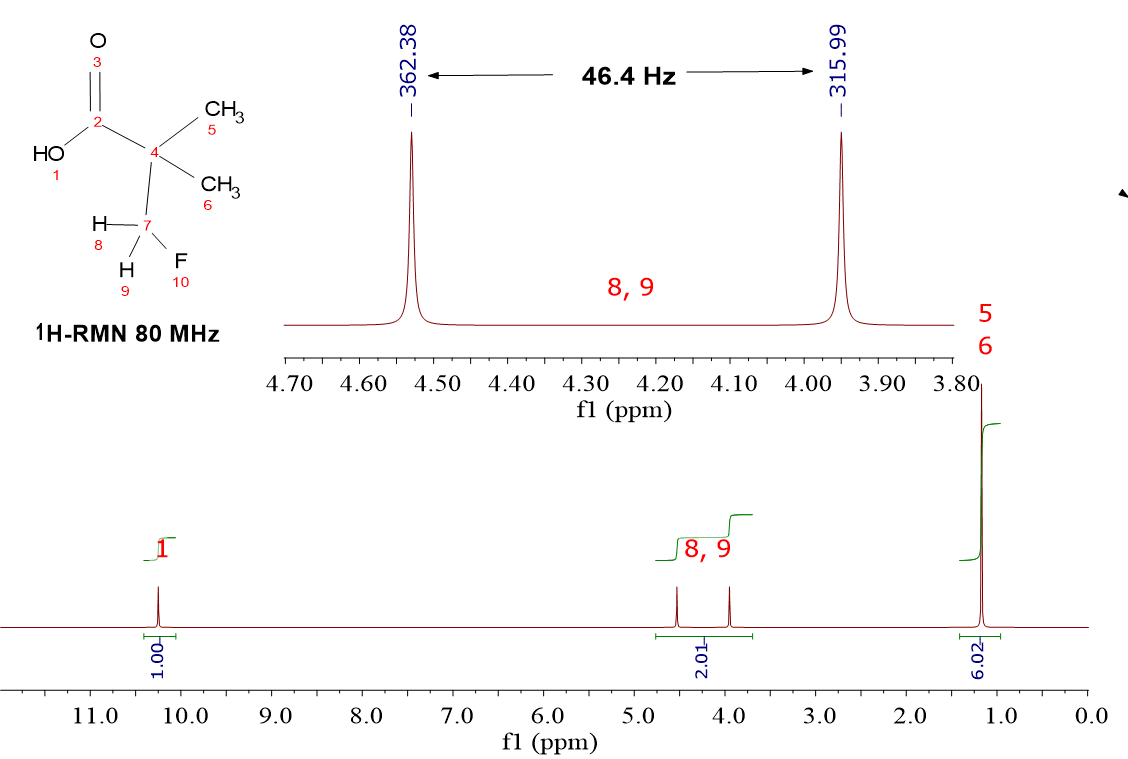 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2
The most obvious difference between the two molecules is the presence of the CH2F group, whose 1H's resonate at a very high chemical shift (4.25 ppm), much higher than the CH3 groups (1.2 ppm). That is due to the proximity of the fluorine, very highly electronegative.
In addition, the signal belonging to the CH2F group shows up as a “doublet” because of the NMR-active 19F that behaves as a tiny magnet. Half of them alligns with the external magnetic field and the other half opposes to it. The 1H's thus end up "feeling" two different external fields and give a “doublet” (N + 1 rule where N = 1, one 19F).
The coupling constant H-C-F at two-bond distance is relatively high (ca. 45 Hz).
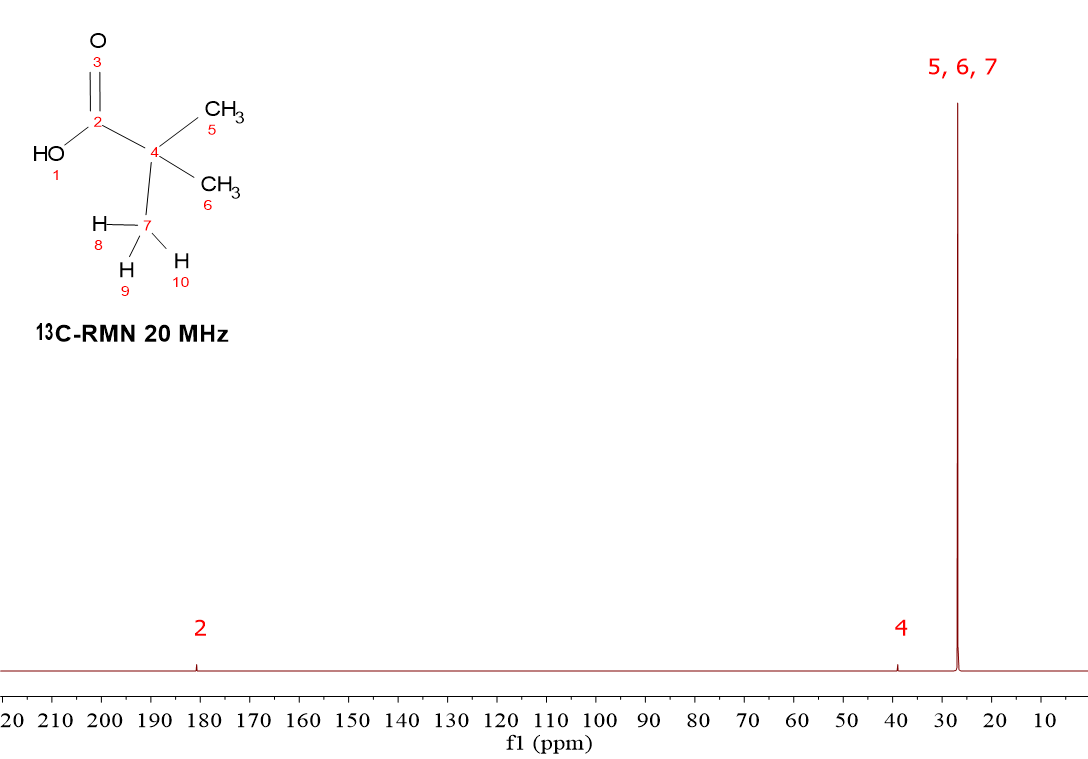 HOOC-C(CH3)3
HOOC-C(CH3)3
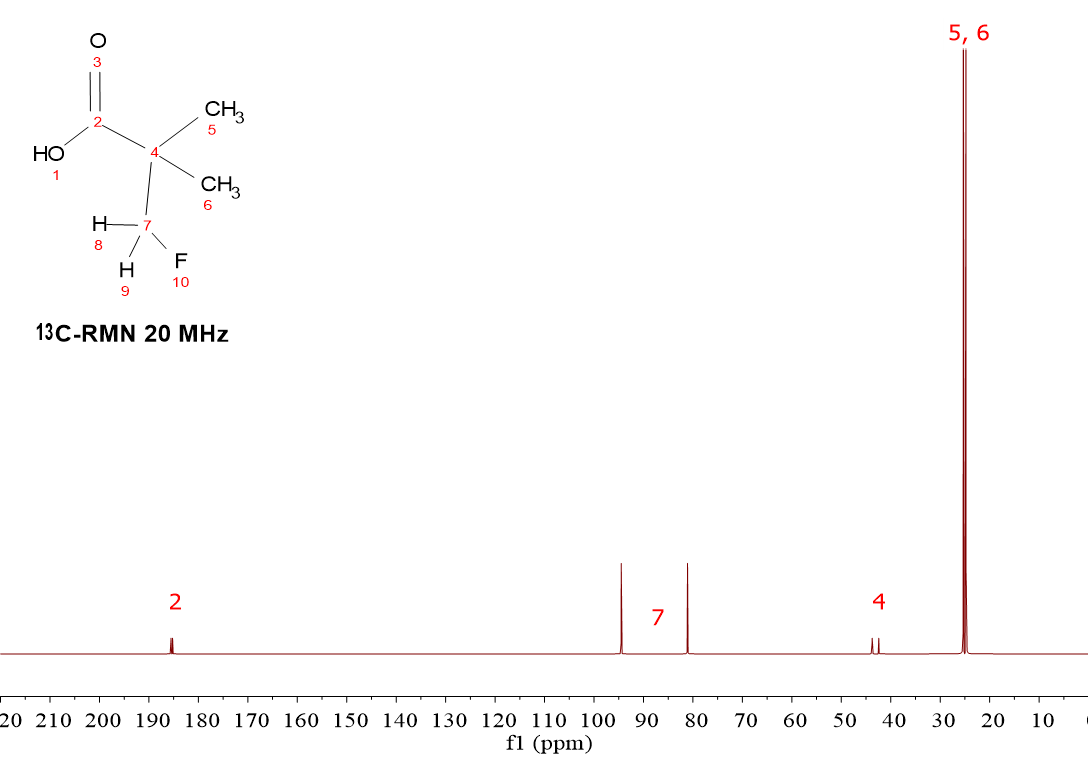 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2
Again the CH2F group makes the difference.
The carbon arbitrarily numbered 7 suffers a heavy increment of its chemical shift due to its direct bonding to 19F whose high electronegativity leaves the carbon naked of electron density.
Besides, the presence of the NMR-active 19F and its half allignment/half opposition to the external magnetic field makes C(7) show up as a "doublet".
Actually, all carbons "feel" two external magnetic fields and all show up as doublets, the magnitude of the splitting being inversely proportional to their distance to the 19F atom.
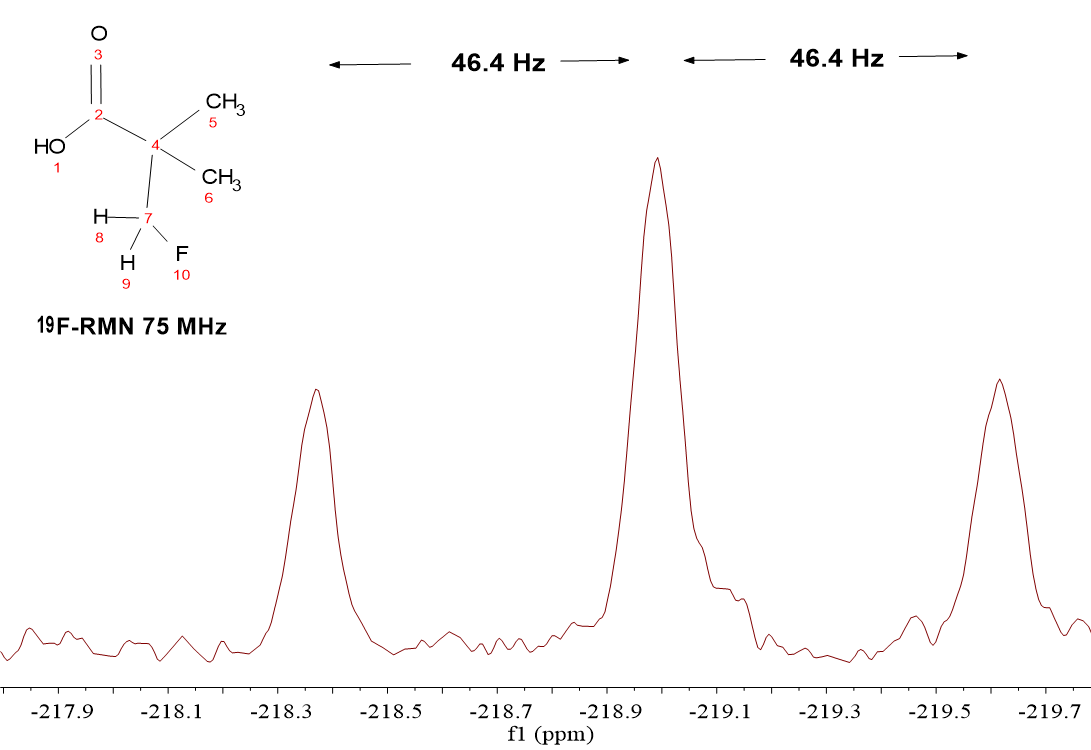 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2
19F bears a gyromagnetic constant close to that of 1H (251.7 and 267.5 · 10^6 rad·s^-1·T^-1, respectively) and 19F and 1H resonate at close frequencies (75 MHz for 19F and 80 MHz for 1H in a field of 1.8 Tesla).
Despite that, one can easily obtain either 19F or 1H spectra.
We can see that the signal coming out from 19F is a “triplet” at a kind of weird chemical shift (-219 ppm) which we won't comment at this moment.
The "triplet" shape is easily explained by the N+1 rule
where N = 2, i.e. the two 1H's of the CH2F group.
The coupling constant is identical to that seen in the 1H-NMR spectrum (vide supra) because the coupling phenomenon is reciprocal.
Had the studied molecule contained a 31P, the result would have been very similar.
Yet, what happens when we consider NMR-active nuclei of low natural abundance?
The best example is 13C. We've seen that 19F and 31P, 100% abundant, make the 1H's signals split.
Does 13C make similar splittings on 1H?
The answer is yes, but only on the 1% of the molecules bearing 13C.
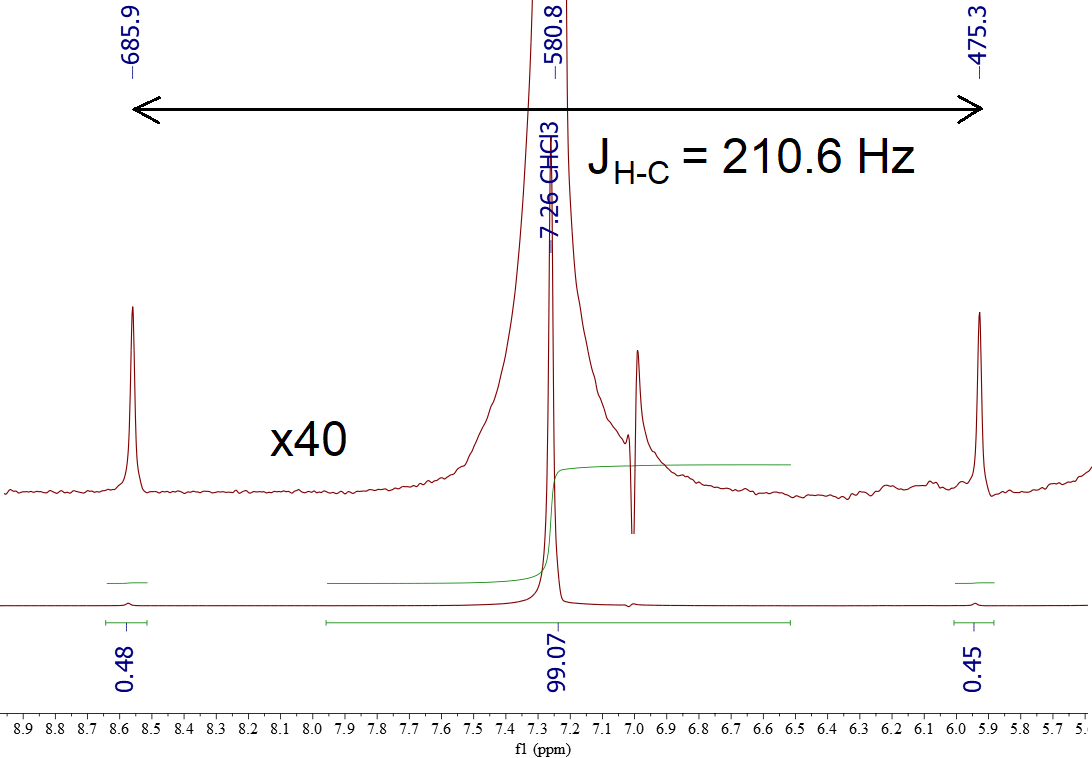
Look at the 1H-NMR spectrum of CHCl3.
If one expands it verticaly, two signals show up flanking the intense one. They are called "13C-satellites" and their integrals are aproximtely 0.5 + 0.5 reltive to ca. 99 intensity assigned to the central signal belonging to the most abundant species 12CHCl3.
Therefore, ca. 1% of the molecules being 13CHCl3 display the expected "doublet" splitting, as 'satellites' of the central signal of 12CHCl3.
The coupling constant is very large (210.6 Hz) because 13C and H are directly bonded and influence each other heavily.
These "13C-satellites" are always present in all 1H signals although they cannot usually be seen unless we perform a huge vertical expansion.
Please, don't be wrong assuming these signals are impurities!!!
 HOOC-C(CH3)3
HOOC-C(CH3)3 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2 HOOC-C(CH3)3
HOOC-C(CH3)3 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2 HOOC-C(CH2F)(CH3)2
HOOC-C(CH2F)(CH3)2