ISOTOPES, THEIR ABUNDANCE AND MAGNETIC PROPERTIES
The isotopes bearing an odd number of protons and/or neutrons possess a magnetic moment and are thus NMR active.
For an isotope, to be "NMR active" means that their nuclei undergo a "precession" movement over themselves when they are introduced into a magnetic field.
The frequency of that "precession" is proportional to the strength of the magnetic field and to a constant different for every isotope, named "gyromagnetic constant".
Therefore, for a magnetic field of a given strength, the nuclei of every isotope will precess with a frequency of their own, different from the other isotopes, in the MHz range.
The interaction of a radio signal, of exactly the same frequency to that of the precessing nuclei, will produce the resonance of only one isotope and its NMR spectrum.
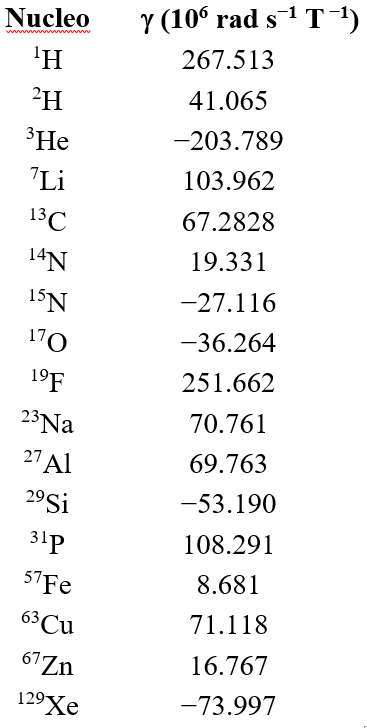
The table contains the gyromagnetic constants of the most common nuclei in NMR. The sign indicates whether a given isotope precesses clockwise or counterclockwise.
For instance, in a magnetic field of 1.8 Tesla, it is seen that 1H's precess at 80 MHz and hence, they resonate and give an 1H-NMR spectrum when irradiated with a radiofrequency of 80 MHz, exactly.
However, in that very same field, other nuclei resonate at their own and different frequencies.
In the case of 13C, the resonance of this isotope occurs at a frequency proportional to the quotient:
“gamma” 13C / "gamma" 1H = 68.283 / 267.513 = 0.256
i.e. at a frequency of de 80 · 0.256 = 20.42 MHz.
The NMR-active isotopes resonate with different radiofrequencies but, at this point, it is necessary to raise the following important question:
Will all isotopes display the very same response intensity?
In other more practical words, let's assume we have 1 mol of hydrogen and 1 mol of carbon:
Will we obtain signals of equal intensity in the 1H- and 13C-NMR spectra?
The answer is a rotund NO due to two reasons.
First of all, the natural abundance of the 1H isotope in 1 mol of hydrogen is 99.99%, whereas that of 13C in 1 mol of carbon is only 1.1%.
Consequently, from that point of view, the 1H signal will be ca. 110 times as intense as that of 13C or, put in another way, the 13C signal will be 110 times MORE DIFFICULT to detect than that of 1H.
Besides, the signal intensity of a given isotope depends on the cube of the gyromagnetic constant.
If one compares 1H (gamma^3 = 19.1·10^6) and 13C (gamma^3 = 0.3·10^6), the signal of the latter is ca. 64 times LESS intense than that of 1H.
Taking into account both factors, the natural abundance and the cube of the gyromagnetic constant, 13C is MUCH MORE DIFFICULT to detect than 1H by a factor of ca. 7,000 times.
In other words, the sensitivity of the NMR detection of a given isotope is very variable and one has to be aware of the latter's abundace and gyromagnetic constant.
In the case of 13C, its lowest intrinsic sensitivity implies larger sample amounts and longer data acquisition times.
Despite that, 13C-NMR is nowadays routinely performed.