RETROSYNTHESIS:
EXAMPLE 1
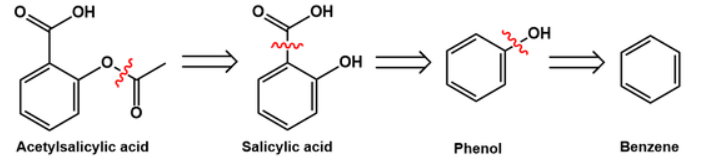
ASPIRIN
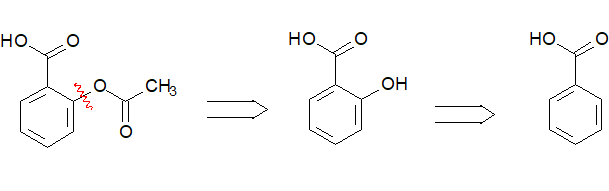
Aspirin is a relatively simple molecule in which, however, one can find many strategic bonds that might be made from simpler precursors.
Strategic bonds are in general those polar bonds that can be constructed starting from smaller molecules.
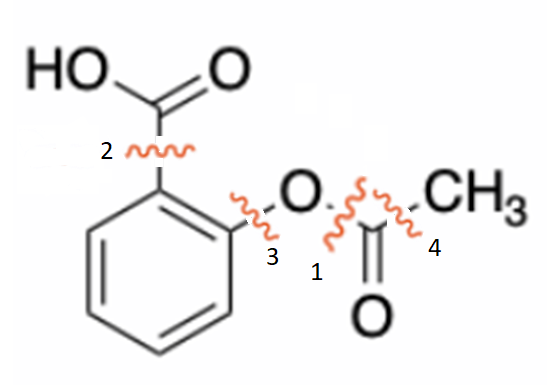
The building of bond 1 would imply the formation of an ester, from the phenol and the appropriate acetyl derivative.
If this is your proposal for the synthesis of aspirin, you then need to have 2-hydroxybenzoic acid available as the precursor and remember the practical way an ester is done (esterification...).
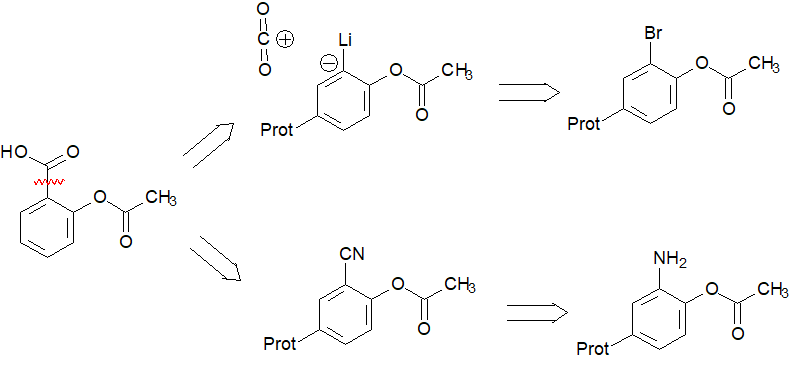 FATAL PROBLEMS:
FATAL PROBLEMS:
1) The acetyloxy group directs mainly para.
2) The formation of an organometallic derivative is not compatible con the carbonyl.
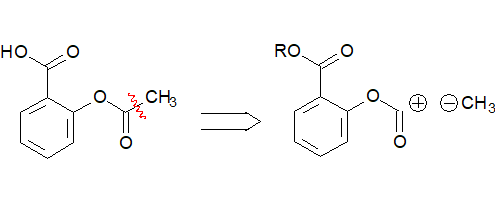
The building of bond 2 would imply the attachment of a carboxylic acid in ortho position to the acetyloxy group.
If this is your proposal for the synthesis of aspirin, you then need to have acetyloxybenzene available as the precursor and remember practical ways to attach the ortho acid group to the benzene ring, without (IMPORTANT) interferring with the acetyloxy group.
The building of bond 3 would imply the ortho-hydroxylation of benzoic acid.
FATAL PROBLEM:
1) The carboxylic group directs meta.
The building of bond 4 would imply the attack of an organometallic to the carbonyl.
FATAL PROBLEM:
1) The carboxylic group will destroy the orgoanometallic or if protected as an ester would definitely react with it.
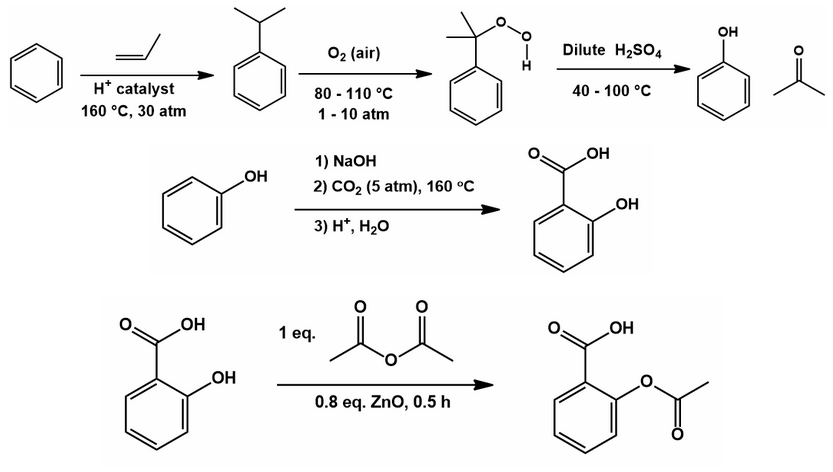
To make a long story short, look at the retrosynthesis of aspirin from benzene and the way it is industrially done:
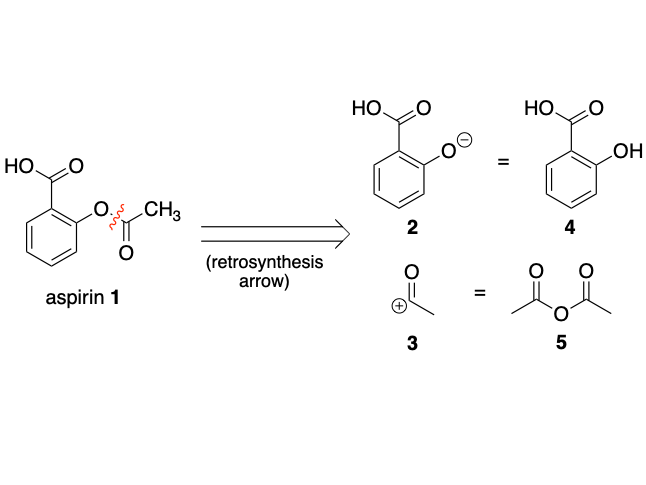
 FATAL PROBLEMS:
FATAL PROBLEMS: