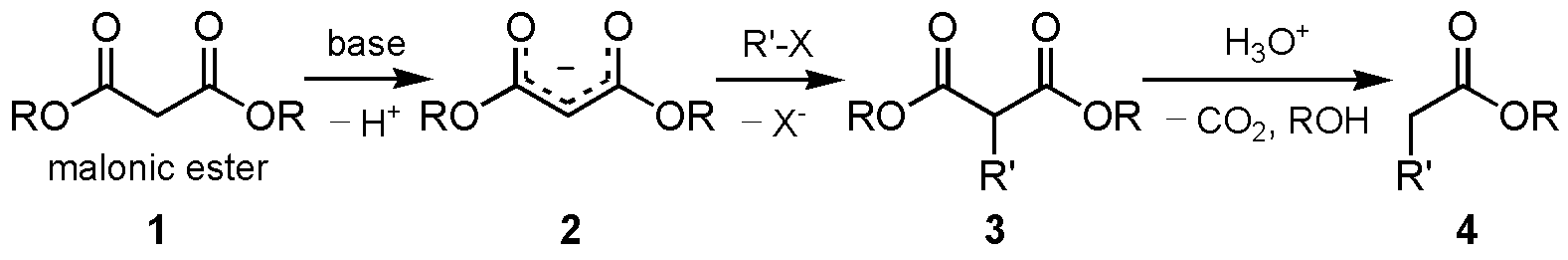MALONIC AND ACETOACETIC SYNTHESIS
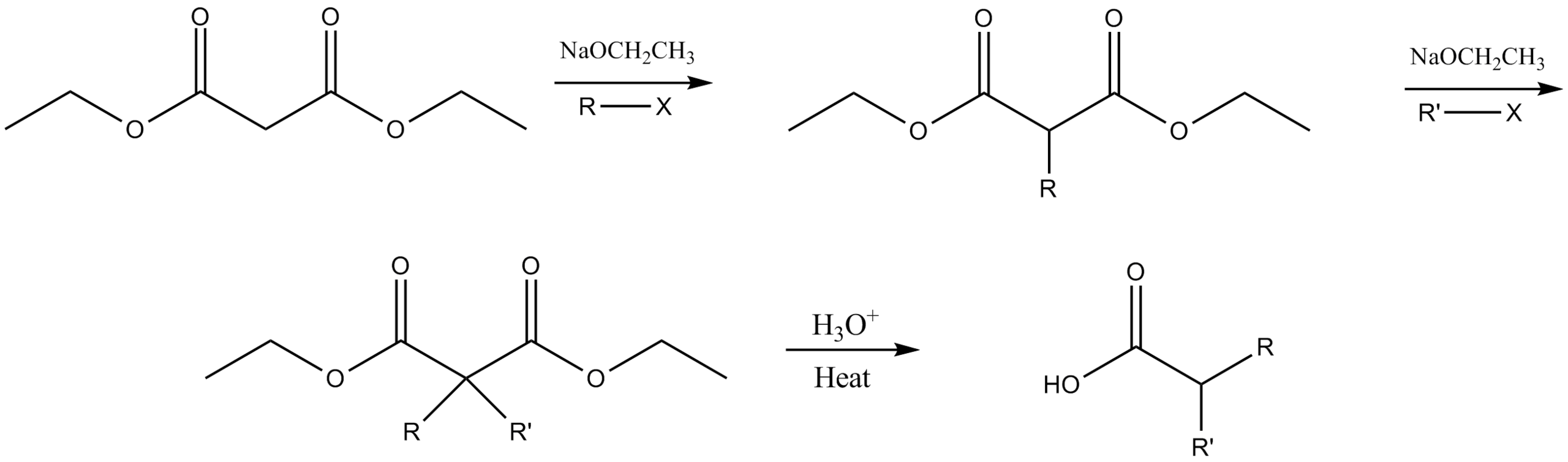
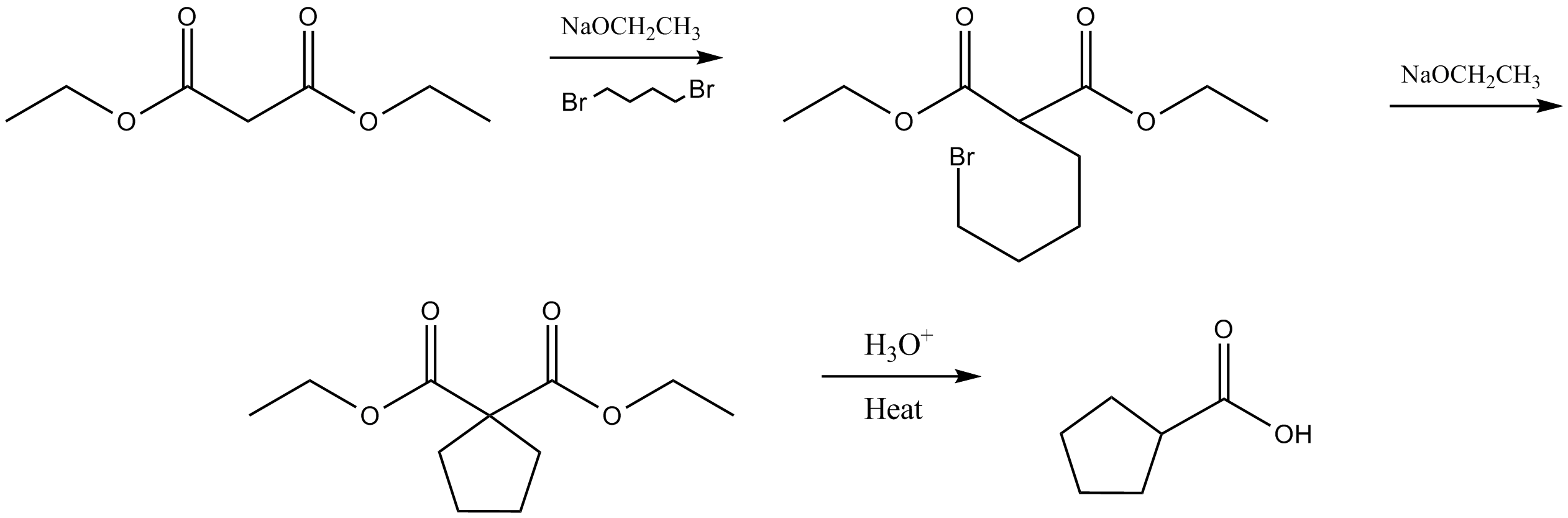
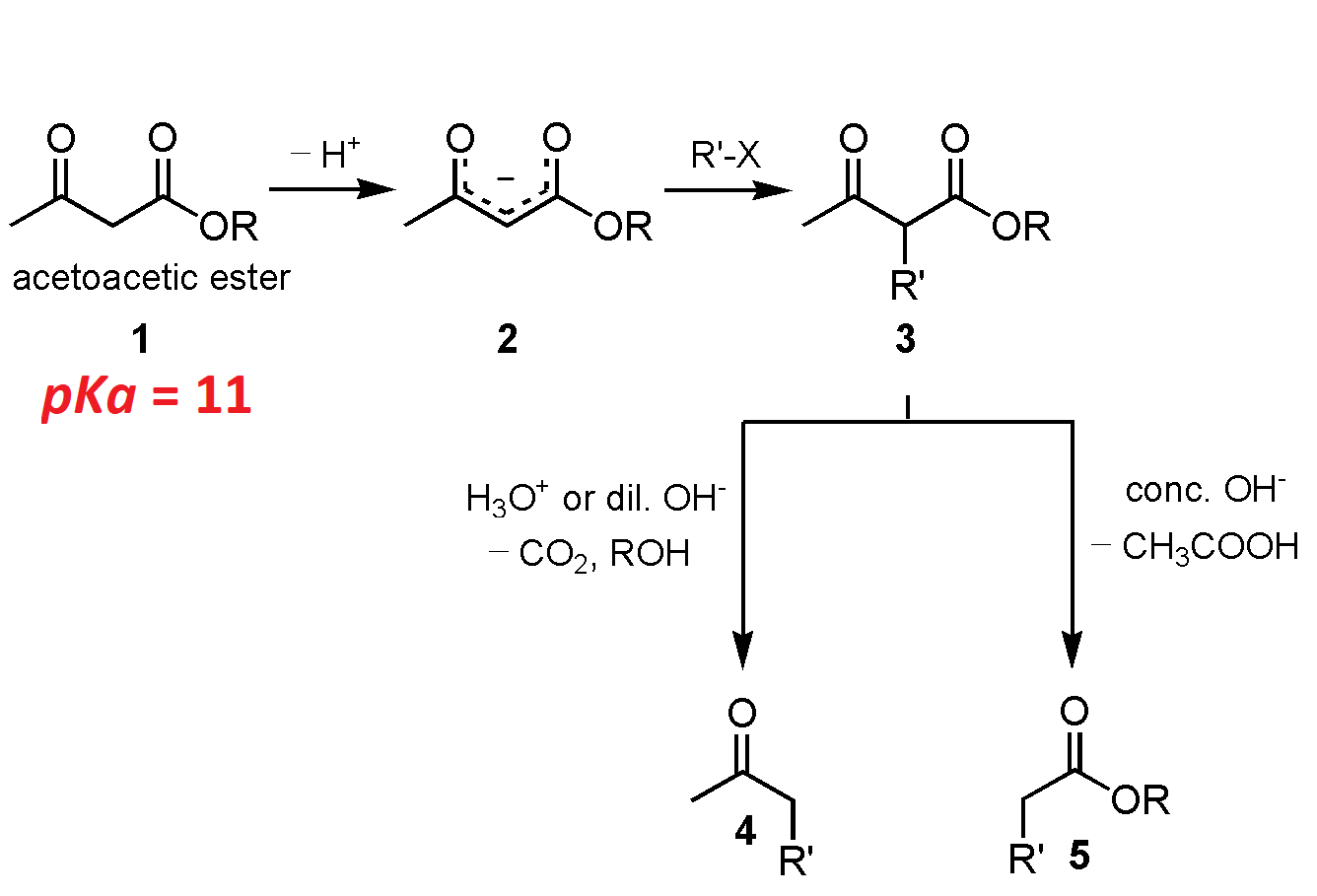
The malonic ester synthesis is a chemical reaction where an ester of malonic acid is alkylated at the carbon in between the carbonyl groups, and then converted to a substituted acetic acid by final hydrolysis and decarboxylation.
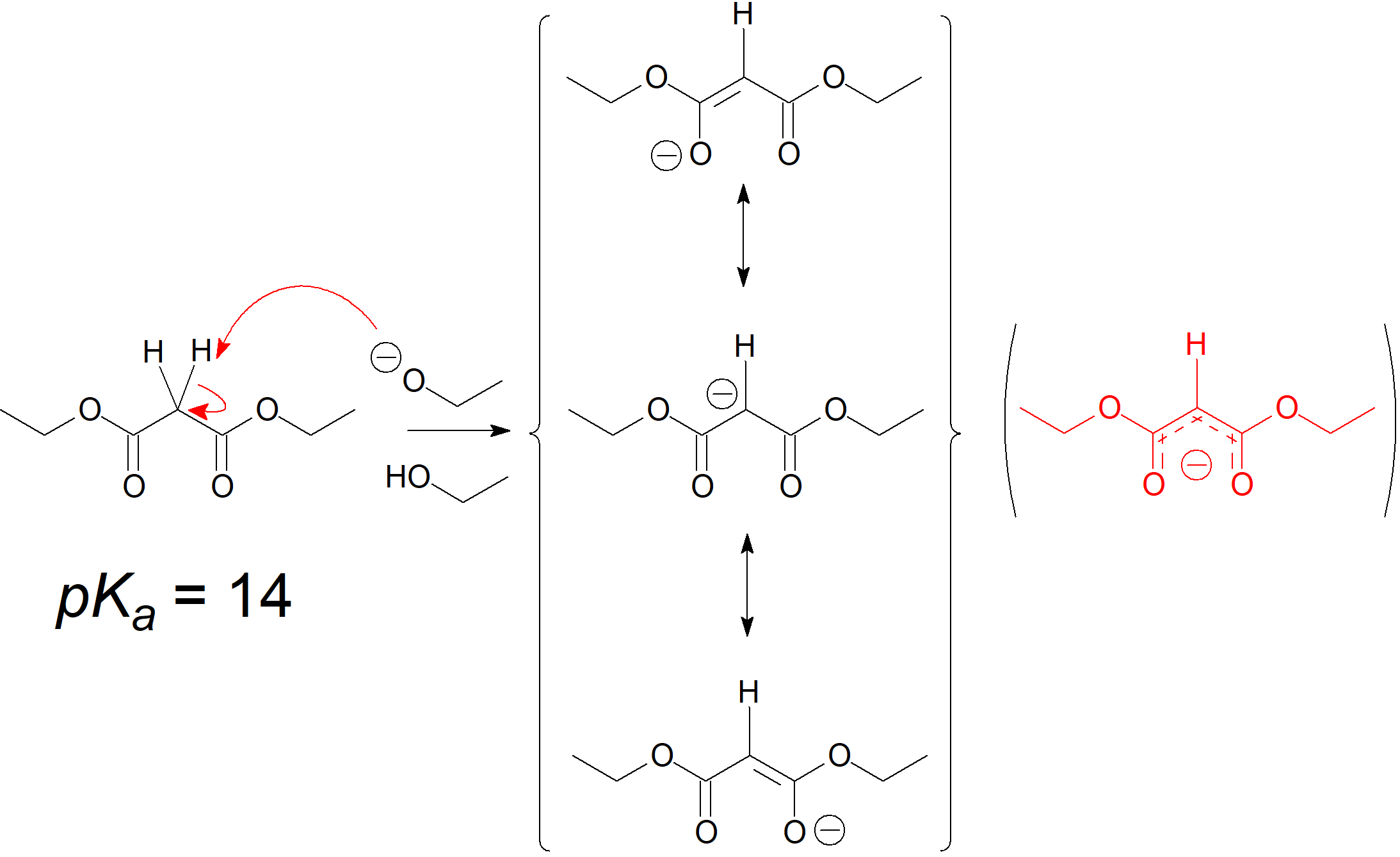
The hydrogens of carbons in between carbonyl groups are quite acidic because the negative charge of the conjugate-base carbanion is stabilized by both C=O groups.
The resulting carbanion is very nucleophilic and can perform a nucleophilic substitution on a compound with a good leaving group like an alkyl halide or sulphonate. The alkylated diester is then saponified and on heating, the dicarboxylate easily loses CO2 rendering an alkylated acetic acid.
Think retrosynthetically!!!
Malonic diester can be considered as a -CH2COOH synthon equivalent.
Do the alkylation twice and the outcome is a dialkylated acetic acid.
The cycloalkyl-carboxylic acids can be obtained this way by using alkyldihalides of different alkyl lengths.
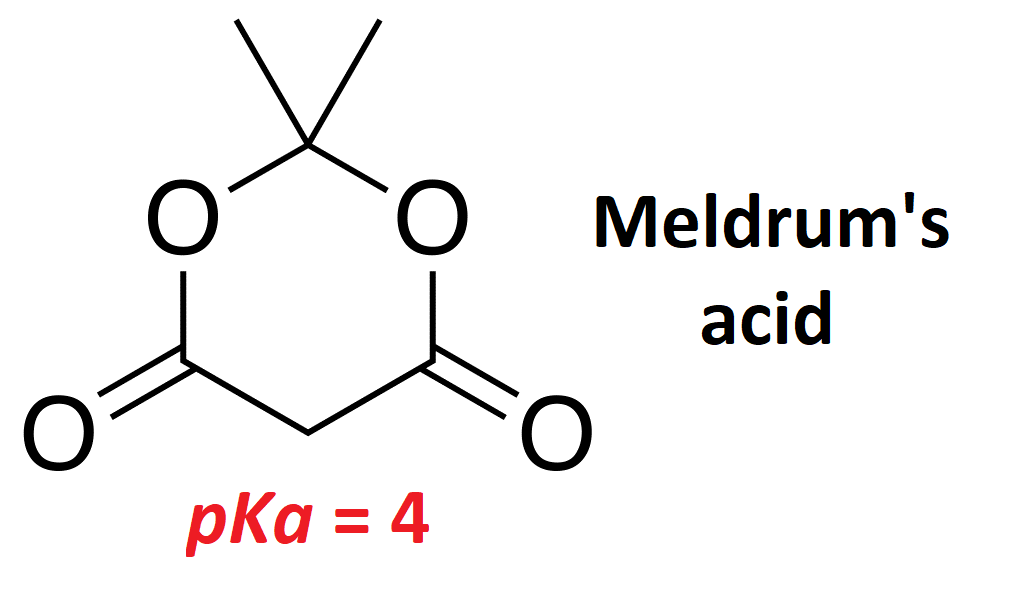
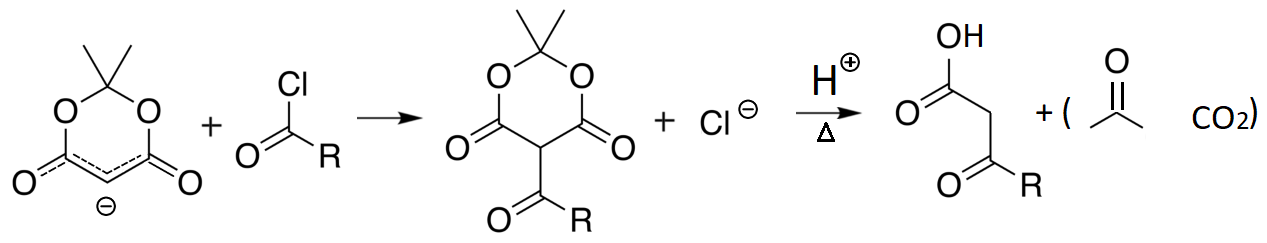
A very interesting malonate-related molecule is Meldrum's acid, named after its discoverer in 1908, that is a malonic diester of acetone hydrate.
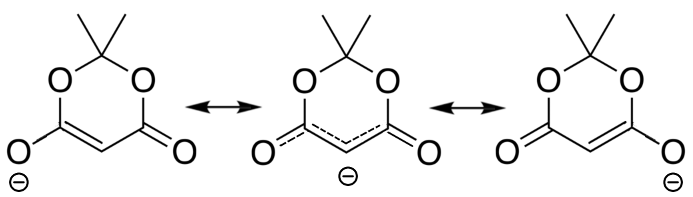
Meldrum's acid is even more acidic than malonate.
Andrew Norman Meldrum (1876, Alloa – 1934, Edinburgh) was a Scottish scientist known for his work in organic chemistry and for his studies of the history of chemistry. It has been claimed that Meldrum's acid 'is the only chemical to be named after a Scotsman.'
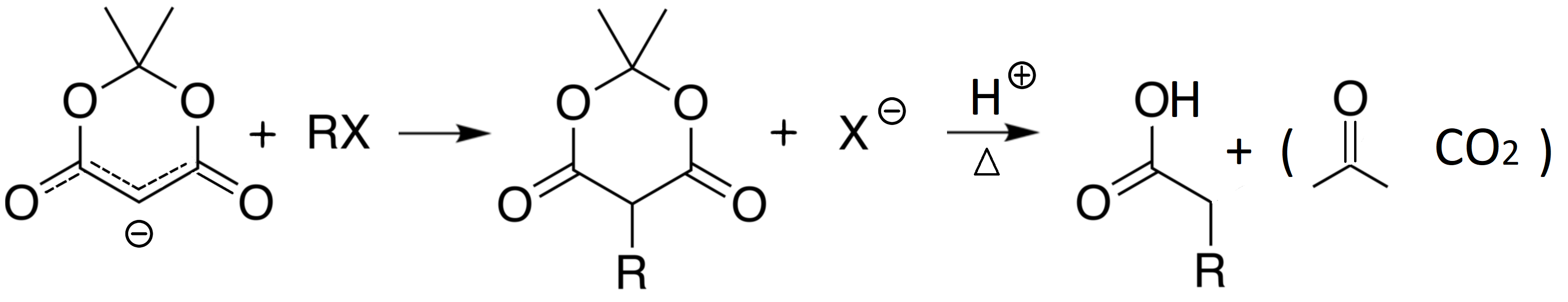
The resulting carbanion is also very nucleophilic and can perform nucleophilic reactions, leading to alkylated or acylated acetic acid after hydrolysis.
The acylacetates obtained from Meldrum's acid can be further used in synthesis because, as it happens in Malonic derivatives and in Meldrum's acid itself, the hydrogens on the carbon in between the carbonyls are relatively acidic and the resulting anion can act in nucleophilic reactions.
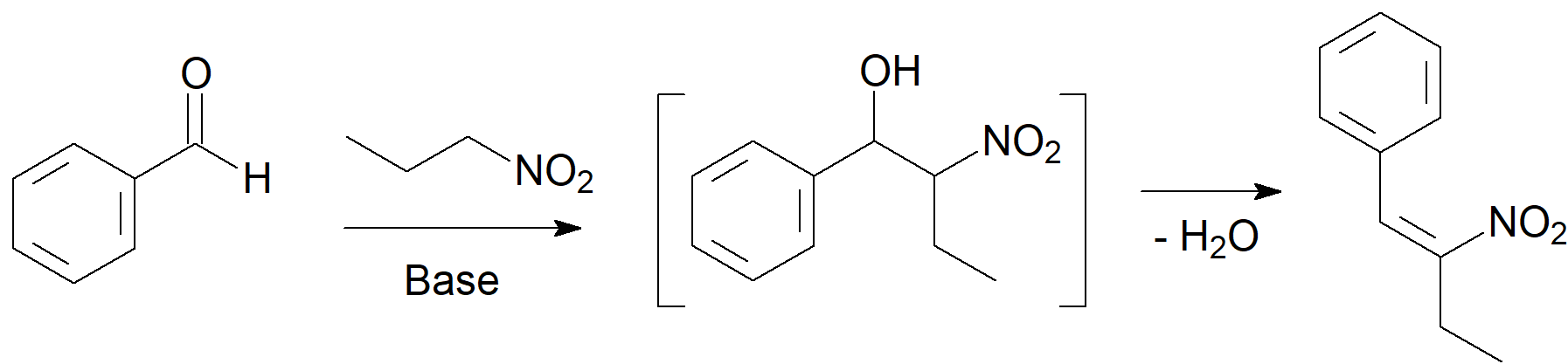
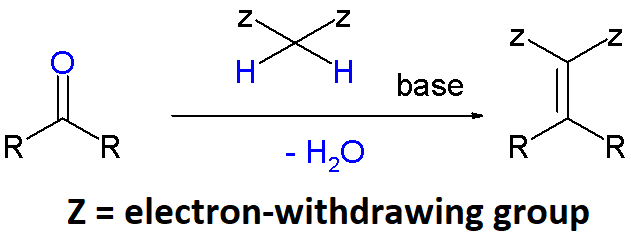
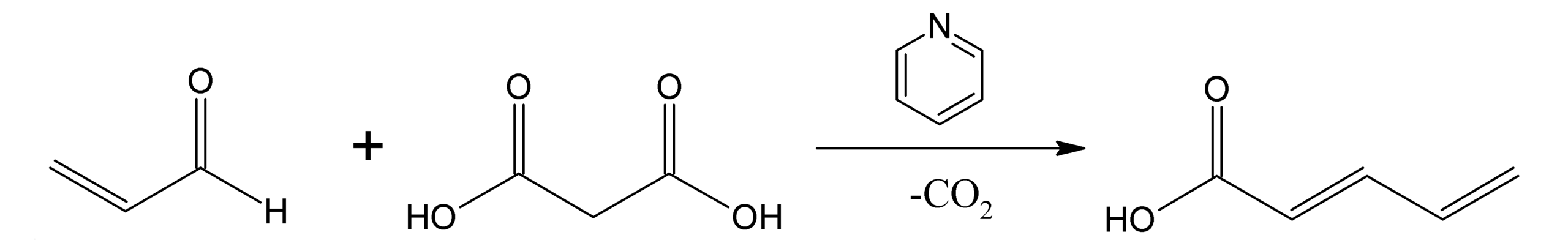
The carbanions from dicarbonyl compounds like malonic esters, Meldrum's acid, acetylacetates and many other related compounds, can react with aldehydes or ketones, lose through a water molecule and render, after (hydrolysis and) decarboxylation, an alpha,beta-unsaturated acid. This is known as the Knoevenagel condensation after the name of its discoverer.
Heinrich Emil Albert Knoevenagel (18 June 1865 – 11 August 1921) was the German chemist who established the Knoevenagel condensation reaction. The Knoevenagel condensation reaction of benzaldehydes with nitroalkanes is a classic general method for the preparation of nitroalkenes, which are very valuable synthetic intermediates.