Using chemical language, one could say that the 'shoplifting' is KINETICALLY CONTROLLED, because we 'steal a given object' very quickly indeed.
In contrast, the big robbery is not kinetically controlled - it implies the planning of slow, complex logistics - but THERMODYNAMICALLY CONTROLLED because it makes you slowly but stably rich.
Could we apply this scheme to the halogenation of alkanes?
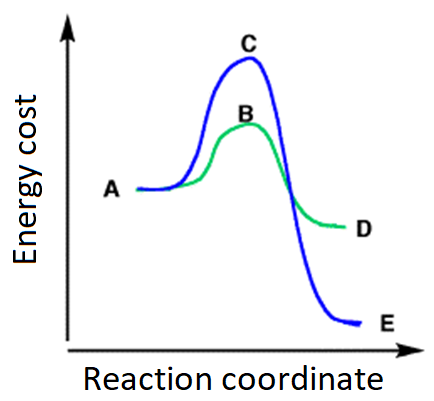
A: X2 + methylbutane
B: Attack to the primary position, much more abundant and accesible - kind of 'shoplifting'.
C: Attack to the tertiary position, unique and less accesible - needs the planning of the big robbery.
D: Primary radical, much more unstable.
E: Tertiary radical, much less unstable.