(UN)STABILITY OF RADICALS
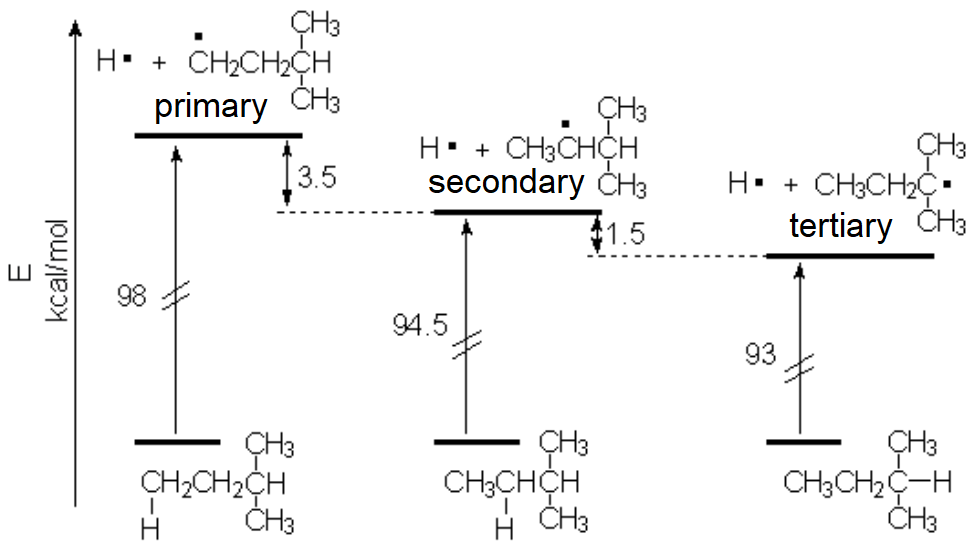
Free radicals - formed by homolytic cleavage of a C-H bond in alkanes - are reactive intermediates whose (un)stability depends on their tertiary (less unstable), secondary or primary structure:
The more substituted a radical, the less unstable it is.
Look at the following experimental data. What can you deduce from them?
A whole lot of things...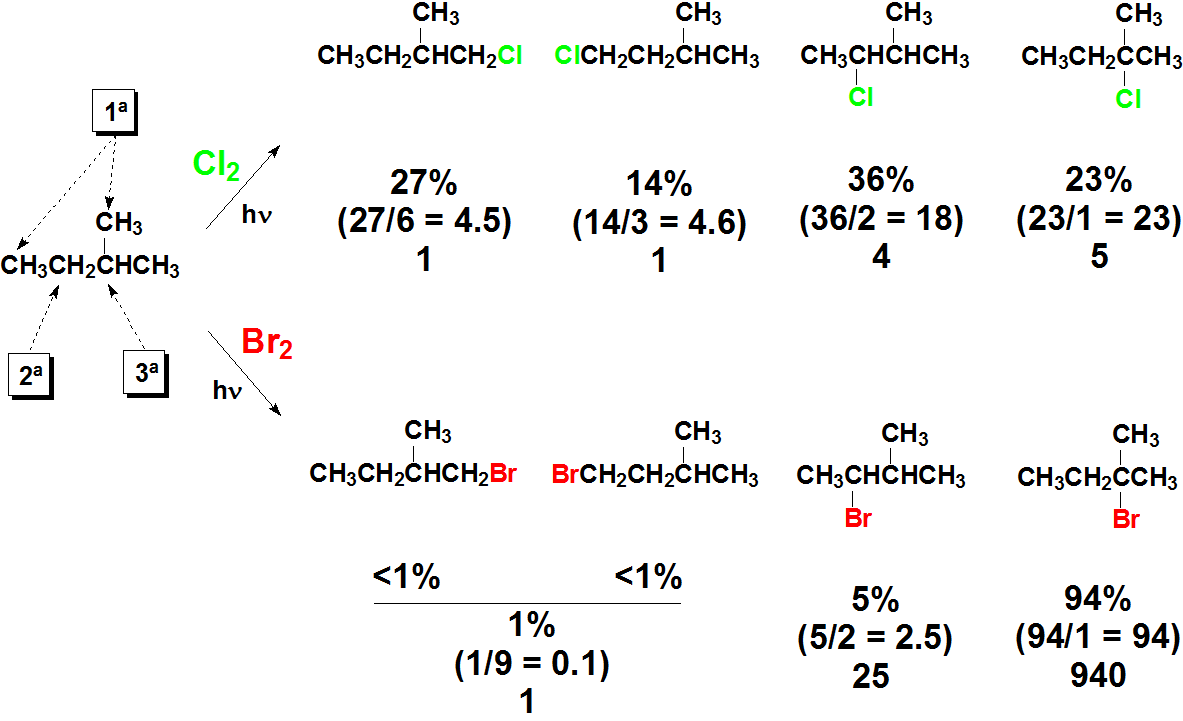
- One obtains mixtures of compounds
- The various positions - two kinds of primary, one secondary and another tertiary - react differently
- The reaction con Br2 looks much more selective
- The tertiary position is the most reactive one, followed by the secondary position. The least reactive is the primary one.
Thanks to these data it was experimentally found that the strength of the C-H's is not the same and depends on the structure.
Experimental results allow us to deduce that the less unstable the radicals, the higher its substitution at the radical center.
Yet, is there a theoretical explanation to this behavior?
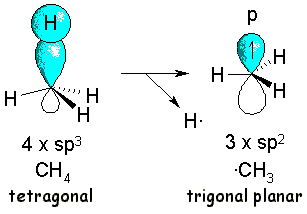
By using pretty sofisticated techniques, researchers have experimentally found that the radical methyl is trigonal planar.
Hence, the homolytical cleavage of a C-H in methane can be explained by a change in hybridization, from sp3 to sp2, the unpaired electron residing in the leftover p orbital.
The central carbon of the ·CH3 radical is electron defficient because it lacks the octet. End of story in the methyl radical.
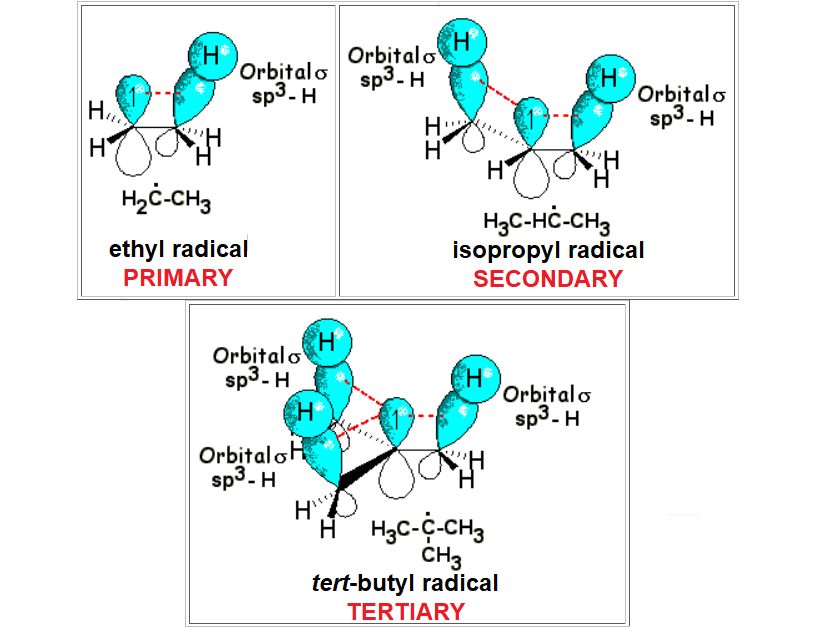
However, if the radical is more complex, there exist possibilities of importing electron density from adjacent carbons thus diminishing the inherent unstability of the radical center.
The neighboring sigma bonds to the radical p orbital can mathematically give rise to a certain degree of distorted, lateral overlapping - reminiscent of a double bond but way less effective.
The defficiency of electron density at the radical center can thus be mitigated to a small extent.
The larger the amount of sigma bonds surrounding the radical center, the larger its stabilization, although always small.
This simple rationale allows us to understand why a tertiary radical is the least unstable of them all.