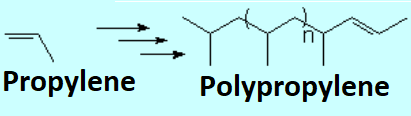
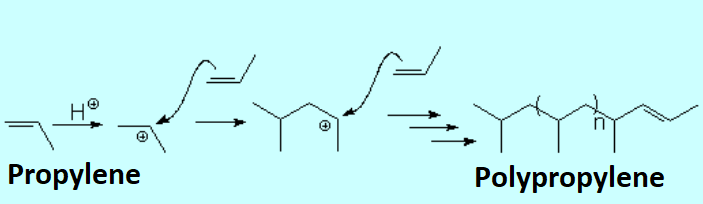
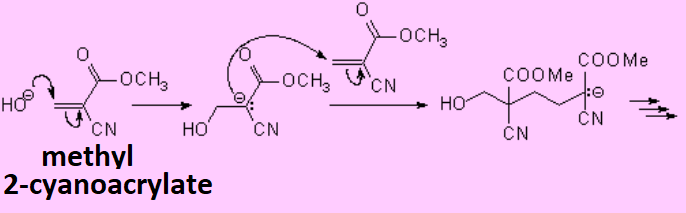
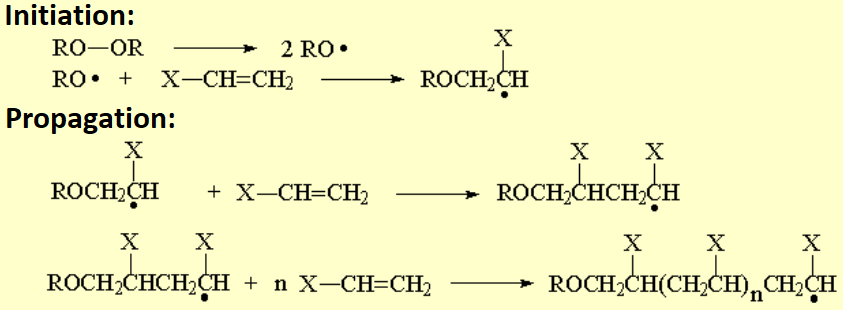
Olefins can react within themselves to render long molecules of variable length.
If two molecules (monomers) bond, one gets a dimer.
If the formed molecules are not very long, we name them oligomers.
A polymer is then built from thousands of monomers.
Polymers are very important from the industrial poit of view because they are profusely used to create almost all objects and tools essential for our everyday life (synthetic fibers for textiles, bottle making, pipes, almost anything...).
Polymerization can be effected by different methods, involving the usual studied reaction intermediates: carbocations, carbanions and radicals. Efficient catalysts have been also developed.
Protonation of an olefin produces an electrophilic carbocation that can be attacked by the "pi" cloud of another molecule.
Electron-poor olefins, i.e. those with electronegative substituents, undergo anionic polymerization.
If a radical initiator is introduced in the reaction, i.e. a peroxide, polymerization proceeds through radicals.
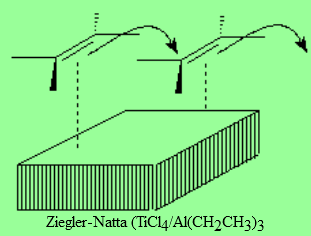
Metal catalyzed polymerization of olefins allows for a control of both length and stereochemistry of the final polymer.
This is paramount to industrially condition the polymer final properties.
The discovery of such metal catalyst was the subject of the Nobel Prize won by their inventors.
Karl Waldemar Ziegler (1898-1973): German chemist, professor at the Halle University, in 1943 was promoted to the direction of the research Max Plank institute of carbon. He discovered the procedure to produce polyethylene at low pressure with linear structure. Nobel Prize of Chemistry (shared with Natta) in 1963.
Giulio Natta (1903-1978): Director of the department of Industrial Chemistry at the Polytechnic Institute in Milan. From 1950 he exclusively devoted his research effeorts to the chemistry of macromolecules. In 1954 he synthetized a highly crystalline polypropilene, based in part in Ziegler's work. He is the father of the idea about tacticity. Nobel Prize of Chemistry (shared with Ziegler) in 1963.